bernthsen acridine synthesis
Home Bernthsen acridine synthesis exhibits the following properties. This video deals with the mechanism of Bernthsen acridine synthesis which is a reaction of diarylamine with carboxylic acid in the presence of zinc chloride.
Synthesis Reactions And Medicinal Uses Of Acridine Pharmaguideline
2 From o-chlorobenzene acid Ullmann synthesis.
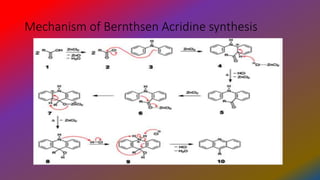
. The Bernthsen acridine synthesis is the chemical reaction of a diarylamine heated with a carboxylic acid or acid anhydride and zinc chloride to form a 9-substituted acridine. Bernthsen-Reaktion Die Bernthsen-Reaktion oder auch Bernthsen-Acridin-Synthese ist eine Namensreaktion der organischen Chemie. The use of polyphosphoric acid will give acridine products at a lower temperature but also with decreased yields.
Formation of 5-substituted acridines by heating diarylamines in organic acids or anhydrides usually in the presence of zinc chloride. Formation of 5-substituted acridines by heatingdiarylamines in organic acids or anhydrides usually in the presence of zincchloride. References Bernthsen A.
Acridine yellow G 40 is prepared. In the modified method the mixture of diphenylamine an aromatic or. Albert TheAcridinesLondon 1951 p 67.
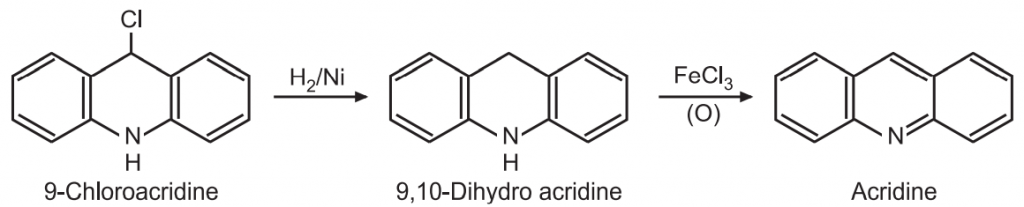
Chemical Synthesis of Acridine 1 Bernthsen acridine synthesis. The synthesis of acridine by heating diphenylamine hydrochloride with benzonitrile is generally known as Bernthsen reaction. Using POCl3 as a catalyst this acid gives 9-chloroacridine.
The use of polyphosphoric acid will give acridine products at a lower temperature but also with decreased yields. Other methods such as heating zinc chloride with N-acyldiphenylamine or a mixture of diphenylamine and acyl chloride have also been used for the synthesis of acridines. The use of polyphosphoric acid will give acridine products at a lower temperature but also with decreased yields.
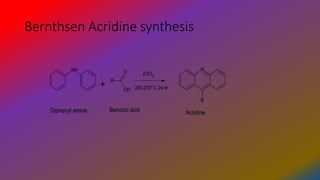
You are able to perform searches and obtain result sets but do not currently have access to the full monographs. Bernthsen acridine synthesis - In the presence of zinc chloride diphenylamine condenses with carboxylic acids to form acridines. Heating diphenylamine to 260 C 10 h with benzoic acid and zinc chloride gave a 48 yield of.
Can Bernthsen acridine synthesis exhibit divisibility. The discovery of acridines as antimalarial and antitumor agents have attracted the attention of organic chemists and thus led to intensive interest in the synthesis of several drugs based on acridine1 2 Acridines are known to be biologically versatile compounds possessing several biological activities3 4 Some of the acridine derivatives bearing a. When diphenylamine is condensed with carboxylic acids in presence of zinc chloride it provides acridines.
Using zinc chloride one must heat the reaction to 200-270 C for 24hrs. Acridine dyes are amino derivatives of acridine. Aniline and o-chlorobenzene acid are condensed to form diphenylamine-2-carboxylic acid.
The Bernthsen acridine synthesis is the chemical reaction of a diarylamine heated with a carboxylic acid or acid anhydride and zinc chloride to form a 9-substituted acridine12Using zinc chloride one must heat the reaction to 200-270 C for 24hrs3 The use of polyphosphoric acid will give acridine products at a lower temperature but also with decreased. Bernthsen Acridine Synthesis Formation of 5-substituted acridines by heating diarylamines in organic acids or anhydrides usually in the presence of zinc chloride. The mechanism for a Bernthsen reaction in which a diarylamine is reacted with a carboxylic acid along with a Lewis acid in Zinc Chloride as well as heat in order to synthesize a 9-substituted.
The Bernthsen acridine synthesis is the chemical reaction of a diarylamine heated with a carboxylic acid or acid anhydride and zinc chloride to form a 9-substituted acridine. The Bernthsen acridine synthesis is the chemical reaction of a diarylamine heated with a carboxylic acid or acid anhydride and zinc chloride to form a 9-substituted acridine. Using zinc chloride one must heat the reaction to 200-270 C for 24hrs.
Acridines are heterocyclic compounds derived from anthracenes formed by two rings fused to a pyridine ring in a central position Scheme 1 also known as dibenzo-pyridine 10-azaantracen and. Using zinc chloride one must heat the reaction to 200-270 C for 24hrs. Media in category Bernthsen acridine synthesis The following 4 files are in this category out of 4 total.
The Bernthsen acridine synthesis is the chemical reaction of a diarylamine heated with a carboxylic acid or acid anhydride and zinc chloride to form a 9-substituted acridine. Bernthsen Reaktion Mechanismus Version 3svg 639 579. 1 2 Using zinc chloride one must heat the reaction to 200-270 C for 24hrs.
AICI3 ZnCl2 forming 9-substituted acridines 20 Bernthsen synthesis. Sie wurde zum ersten Mal im Jahr 1878 von August Bernthsen 18551931 veröffentlicht. The condensation reaction of diphenylamine with 2-oxo-2H-substituted chromen-4-yl acetic acid in presence of anhydrous zinc chloride afford 4-acridine-9-ylmethyl-2H-substituted chromen-2-one.
Bernthsen synthesis antimicrobial activities and cytotoxicity of acridine derivatives. 3 The use of polyphosphoric acid will give acridine products at a lower temperature. Bernthsen acridine synthesis exhibits divisibility.
1 2 Using zinc chloride one must heat the reaction to 200-270 C for 24hrs. They can be produced in a straightforward manner by a Friedel-Crafts reaction the Bernthsen acridine synthesis 218 which couples diaryl amines such as diphenylamine with a carboxylic acid such as benzoic acid in the presence of a Lewis acid zinc chloride is a typical reagent. BERNTHSEN Acridine Synthesis In the Bernthsen synthesis diphenylamine 61 and carboxylic acids form 9-substituted acridines 62 Scheme 31.
Es handelt sich um eine der ersten Acridin-Synthesen durch Erwärmung von Diphenylaminen mit Benzonitril. Diphenylamine reacts with carboxylic acids in the presence of Lewis acids eg. Albert The Acridines London 1951 p 67.
The synthesized compounds were characterized by spectral studies. From o-chlorobenzene acid - A diphenylamine-2-carboxylic acid is formed by condensing aniline and o-chlorobenzene acid. Bernthsen reaction has been carried out under microwave irradiation in the presence of p-TSA 10 mol as catalyst in a solventless reaction.
Bernthsen acridine synthesis can be divided into things called the. Albert Heterocyclic Compounds4502 1952.
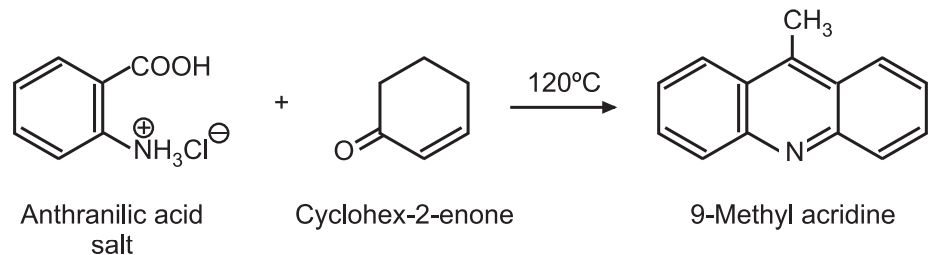
Synthesis And Reactions Of Acridine Solution Pharmacy
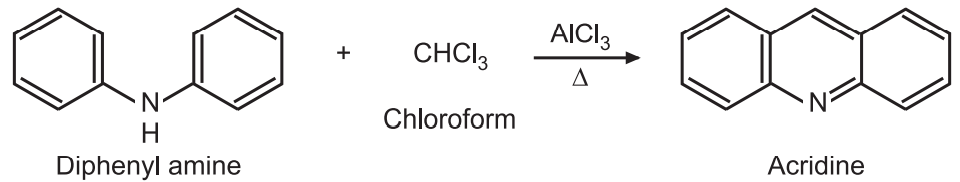
Synthesis And Reactions Of Acridine Solution Pharmacy

Bernthsen Acridine Synthesis Chemistrian

Bernthsen Acridine Synthesis Wikiwand

Mechanism Of Bernthsen Acridine Synthesis Youtube
File Bernthsen Reaktion Mechanismus Version 4 Svg Wikimedia Commons
Synthesis Reactions And Medicinal Uses Of Acridine Pharmaguideline
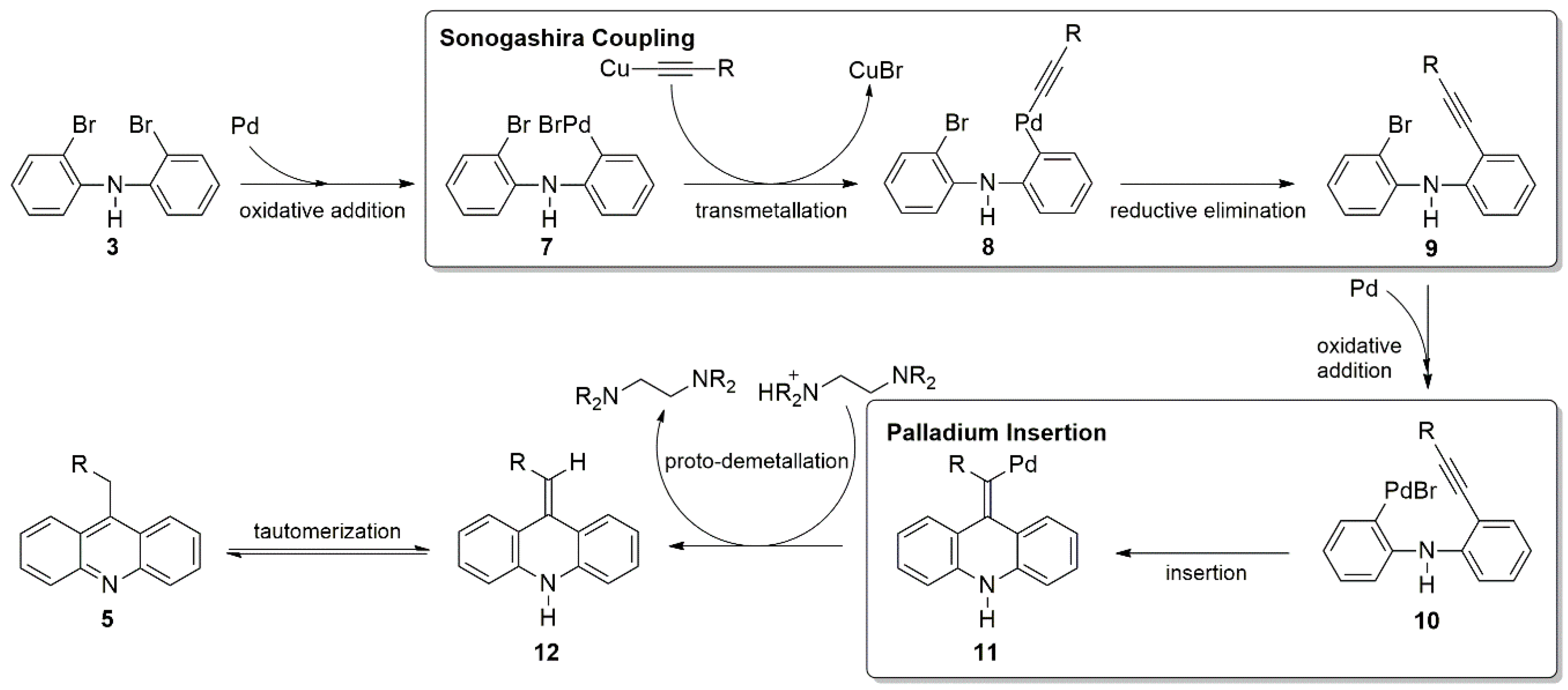
Molecules Free Full Text Synthesis Of Acridines Through Alkyne Addition To Diarylamines Html

Bernthsen Acridine Synthesis Chemistrian

Acridine Preparation Bernthsen Acridine Synthesis And Two Mcq By Dr Tanmoy Biswas Youtube
Synthesis Reactions And Medicinal Uses Of Acridine Pharmaguideline
Synthesis Reactions And Medicinal Uses Of Acridine Pharmaguideline
Bernthsen Acridine Synthesis Chemistrian

Bernthsen Acridine Synthesis Mechanism Organic Chemistry Youtube
Synthesis And Reactions Of Acridine Solution Pharmacy

Acridine An Overview Sciencedirect Topics
Synthesis Reactions And Medicinal Uses Of Acridine Pharmaguideline

File Bernthsen Reaktion Mechanismus Version 4 Svg Wikimedia Commons
0 Response to "bernthsen acridine synthesis"
Post a Comment